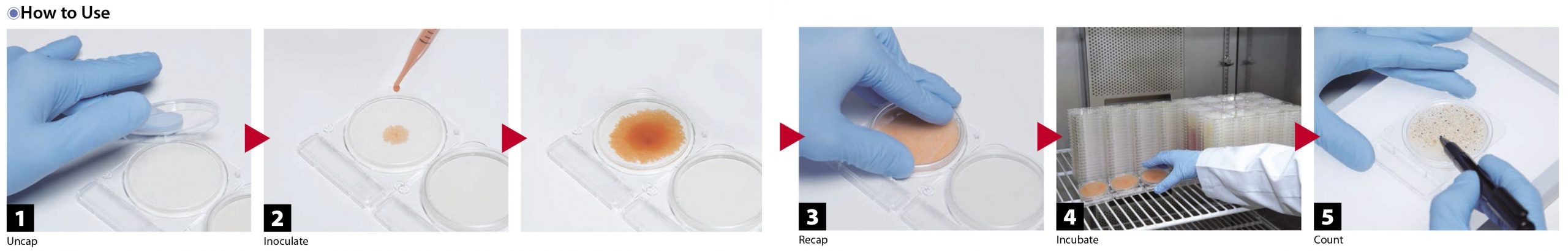
Compact Dry TC (Total Viable Count)
Refers to all microorganisms present in a sample which can tolerate the presence of oxygen and does not have specific growth requirements typically not included in the formulation of general purpose media. In accordance to Food and Drug Administration’s (FDA) regulation, Total Count is considered as one of the release parameters for food (frozen, chilled, precooked, and prepared food), pharmaceutical, and nutraceutical samples.
CompactDry TC was verified for the 24 hours detection using the matrix of raw meats against the reference method.
Procedures